
(ii) that would be produced if the dehydration reaction went to completion.(i) that are actually produced in the experiment and measured in the gas collection tube and.(a) Calculate the number of moles of C2H4(g)
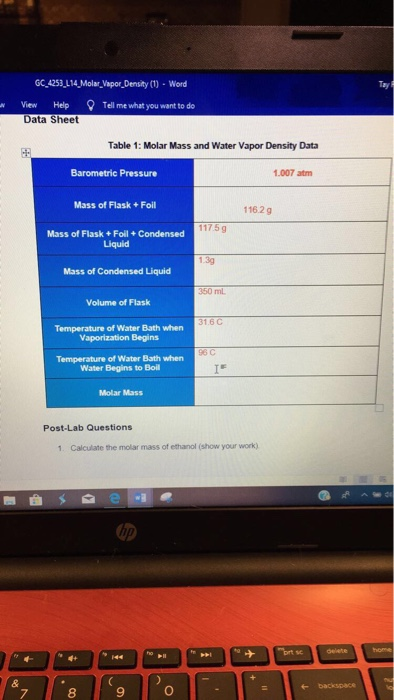
(The vapor pressure of water at 305 K is 35.7 torr.) When the reaction stopped, the volume of collected gas was 0.0854 L at 0.822 atm and 305 K.
The student heated the test tube gently with a Bunsen burner until all of the C H OH(l) 2 5 evaporated and gas generation stopped. The equation for the dehydration reaction is given below.Ī student added a 0.200 g sample of C2H5OH(l) to a test tube using the setup shown above. A setup for the lab synthesis is shown in the diagram above. The QuestionĮthene, C2H4(g) (molar mass 28.1 g/mol), may be prepared by the dehydration of ethanol, C2H5OH(g) (molar mass 46.1 g/mol), using a solid catalyst.
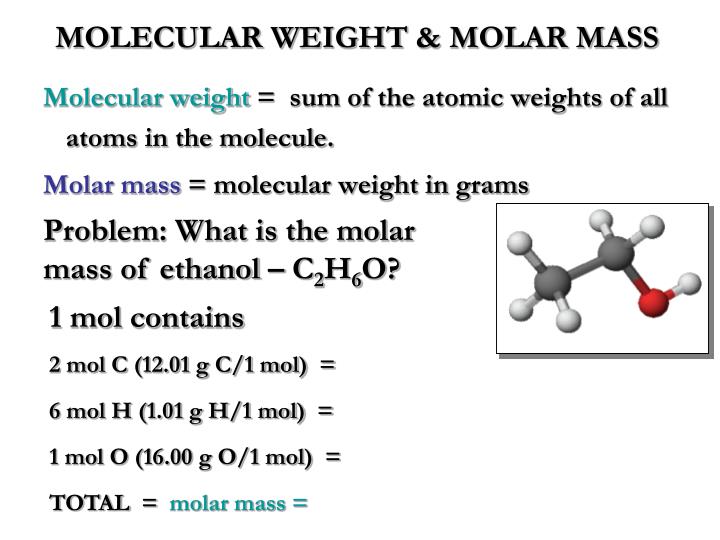
Molar mass ethanol how to#
In this question, students are expected to know how to analyze the experimental data that is given and show an understanding of thermodynamics. #100 color(red)(cancel(color(black)("g solution"))) * "11.52 g ethanol"/(25.03 color(red)(cancel(color(black)("g solution")))) = "46.On average students scored 3.88/10 points on this question while only 1% of students got a perfect score. You can use the known composition of the sample to figure out how many grams of ethanol you'd ge for #"100 g"# of this solution Use the molar masses of the two compounds to convert the number of moles to grams. #chi_"ethanol" = n_"ethanol"/"1 mole" implies n_"ethanol" = 0.25 * "1 mole" = "0.25 moles"#Ĭonsequently, you can say that this sample contains #0.75# moles of water.

Now, you can use the mole fraction of ethanol to say that the number of moles of ethanol present in this sample is equal to #color(darkgreen)(ul(color(black)("% ethanol by mass = 46%")))#Īlternatively, you can start by picking a sample of this solution that contains exactly #1# mole solute and of solvent. Since this represents the mass of ethanol present in #"100 g"# of solution, you can say that the percent concentration by mass of ethanol is Plug this into equation #color(darkorange)((2))# to find Use equation #color(darkorange)((1))# to write Now all you have to do is to solve this system of two equations with two unknowns. Therefore, the mole fraction of ethanol can be rewritten as-for the sake of simplicity, I won't add any units #chi_ "ethanol" = n_"ethanol"/(n_"ethanol" + n_"water")#Īt this point, you must use the molar masses of ethanol and of water to express the mole ratio of ethanol in terms of #m_"ethanol"# and #m_"water"#. You also know that the mole fraction of ethanol, which is defined as the ratio between the number of moles of ethanol and the total number of moles present in the solution, is equal to #0.25#. #m_ "solution" = m_ "ethanol" + m_ "water"# Now, you know that the mass of this sample will be equal to the mass of the ethanol, the solute, and the mass of the water, the solvent. To make the calculations easier, pick a #"100-g"# sample of this solution. I'll show you two methods that you can use to solve this problem.Īs you know, a solution's percent concentration by mass tells you the number of grams of solute present for every #"100 g"# of solution.


 0 kommentar(er)
0 kommentar(er)
